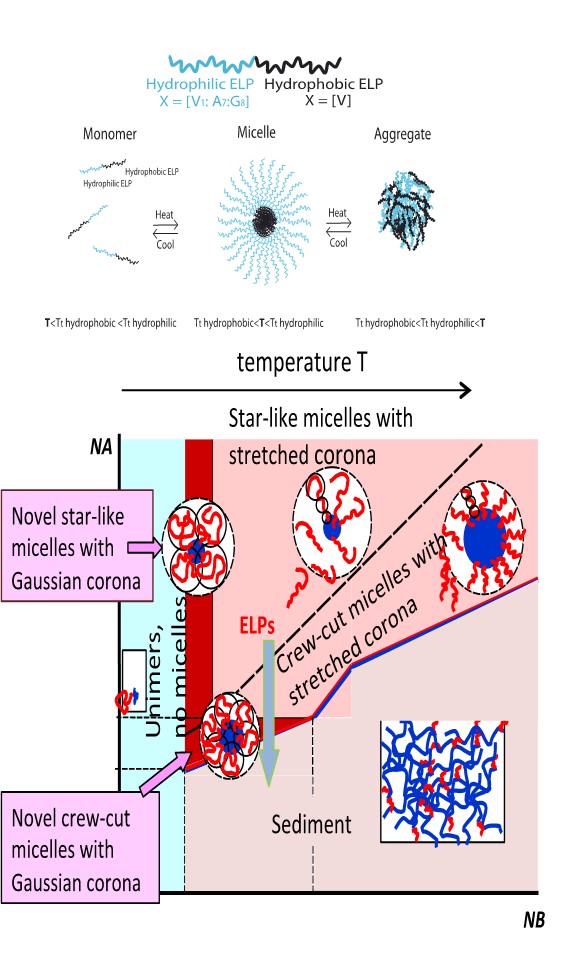
Diblock copolymers
in
selective solvents self-assemble in
aggregates,
called micelles. The insoluble
blocks aggregate in the micellar core,
protected by corona formed by stretched solvated blocks. The structure
of elastin-like
polypeptide (ELP) repeat
unit suggests
that
surface tension at the core-corona boundary could
be
considerably reduced compared to surface tension in conventional micelles
formed by synthetic copolymers. Diblock copolymers
with surface tension below thermal
energy kT per chain
and with relatively short
blocks form
novel micelles with almost
unstretched coronas. The
theoretical
predictions are in good agreement with experimental observations of the micellization
temperature and hydrodynamic radius
of the micelles
that
is consistent
with the unstretched
state of corona
blocks.
This study is important for
the design of polypeptide
carriers with desired size
and properties.