 Pb(Hf0.2Zr0.2Ti0.2Nb0.2X0.2)O3, a high-entropy perovskite, undergoes an entropy-driven phase transformation when X=Mn while X=Al always contains minor second phases in bulk ceramics. Thin films with X=Al show a narrow ferroelectric hysteresis loop and relaxor-like characteristics, i.e. a high dielectric permittivity of ~2000 and low dielectric loss. These are the characteristics needed for device applications.
Pb(Hf0.2Zr0.2Ti0.2Nb0.2X0.2)O3, a high-entropy perovskite, undergoes an entropy-driven phase transformation when X=Mn while X=Al always contains minor second phases in bulk ceramics. Thin films with X=Al show a narrow ferroelectric hysteresis loop and relaxor-like characteristics, i.e. a high dielectric permittivity of ~2000 and low dielectric loss. These are the characteristics needed for device applications.
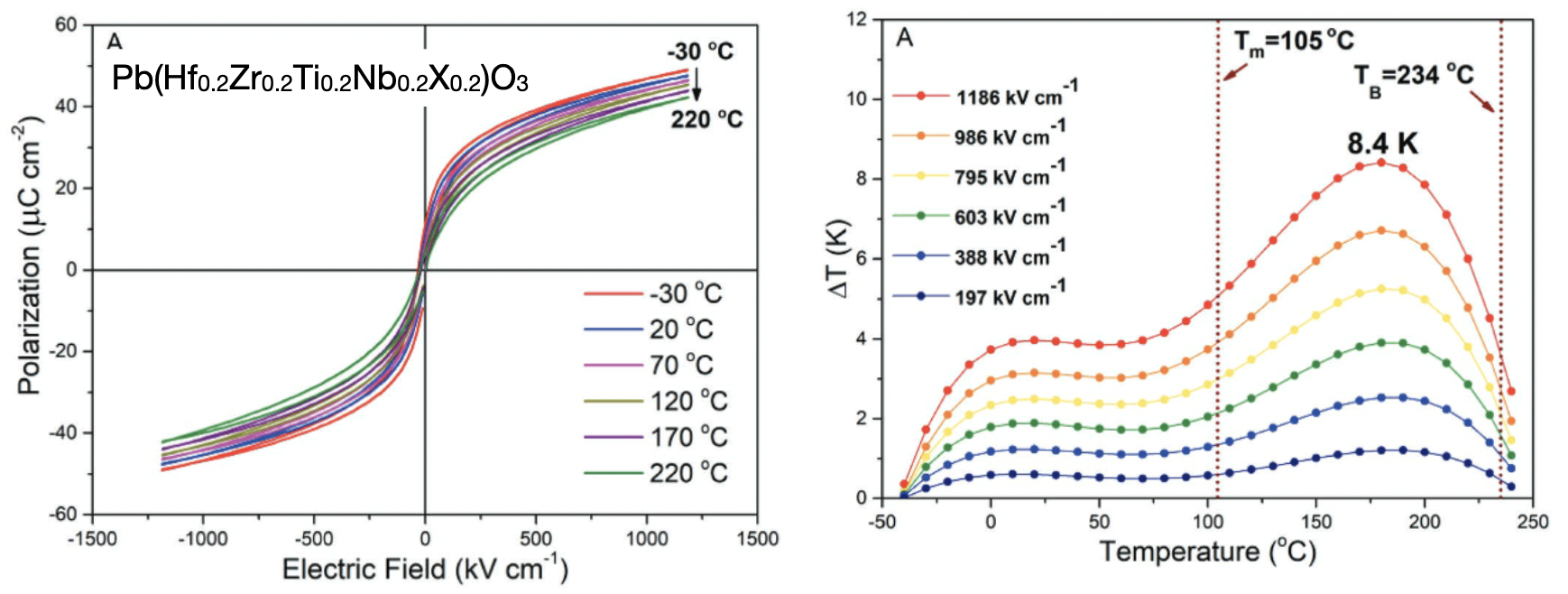
Indirect measurements (based on Maxwell relations) yield a electrocaloric temperature change of 8.4 K at 180°C under an applied electric field of 1186 kV cm−1. The temperature changes in this initial example of a high-entropy electrocaloric oxide are already comparable to those of other oxide-based materials. The huge design space available for optimization of high-entropy formulations now offers opportunities to exceed known electrocalorics in terms of both size of response and operating temperature range.