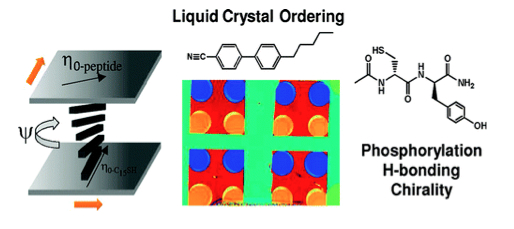
IRG 3 has examined the orientational ordering of nematic liquid crystals (LCs) supported on organized monolayers of dipeptides with the goal of understanding how peptide-based interfaces encode intermolecular interactions that are amplified into supramolecular ordering. By characterizing the orientations of nematic LCs (4-cyano-4′-pentylbiphenyl and TL205 (a mixture of mesogens containing cyclohexane-fluorinated biphenyls and fluorinated terphenyls)) on monolayers of l-cysteine-l-tyrosine, l-cysteine-l-phenylalanine, or l-cysteine-l-phosphotyrosine formed on crystallographically textured films of gold, we conclude that patterns of hydrogen bonds generated by the organized monolayers of dipeptides are transduced via macroscopic orientational ordering of the LCs. This conclusion is supported by the observation that the ordering exhibited by the achiral LCs is specific to the enantiomers used to form the dipeptide-based monolayers. The dominant role of the −OH group of tyrosine in dictating the patterns of hydrogen bonds that orient the LCs was also evidenced by the effects of phosphorylation of the tyrosine on the ordering of the LCs. Overall, these results reveal that crystallographic texturing of gold films can direct the formation of monolayers of dipeptides with long-range order, thus unmasking the influence of hydrogen bonding, chirality, and phosphorylation on the macroscopic orientational ordering of LCs supported on these surfaces. These results suggest new approaches based on supramolecular assembly for reporting the chemical functionality and stereochemistry of synthetic and biological peptide-based molecules displayed at surfaces.