Surfactant adsorption at solid-liquid interfaces is important in many industrial processes, including corrosion inhibition, dispersion stabilization, and lubrication. Furthermore, surfactant adsorption may provide novel and exciting means to guide soft materials to self-assemble into a myriad of tailored shapes. Recently, PCCM researchers have made a breakthrough in elucidating the physical mechanisms behind surfactant self-assembly on a graphite surface [1]. Their simulations reveal that graphite-hydrocarbon chain interactions favor specific molecular orientations along the crystallographic directions, leading to the formation of oriented hemimicellar aggregates as observed in previous experiments [2] from PCCM's IRG2. The results suggest that manipulating the structure of the graphite surface can be employed to tune the orientational order of the hemimicellar aggregates
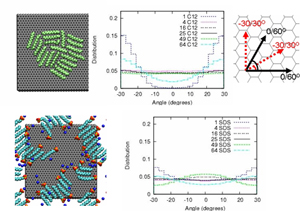
Top left: snapshot of a C12 aggregate on graphite. Top middle: orientational distribution for varying surface coverage. Top right: schematic of the orientations in the middle panel. Bottom left: snapshot of an SDS aggregate on graphite. Bottom right: orientational distribution for varying surface coverage. Both C12 and SDS molecules display strong orientational bias the single molecule level, while bias disappears at intermediate coverages only to re-emerge at complete filling.