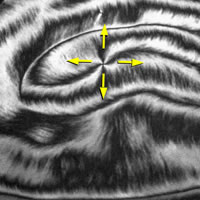
Some molecules, such as water, are electric dipoles, having excess negative charge on one part (the oxygen in H2O) and excess positive charge on another part (the hydrogens in H2O). In liquid water these +/- dipoles are randomly oriented by the thermal motion of the molecules, but in some forms of ice and many other crystals the dipoles orient in a uniform direction and the frozen material becomes ferroelectric, i.e., acquires an overall polarization. Scientists at the LCMRC have created a new family of molecules that can become spontaneously ferroelectric in a phase that is fluid. The image shows the texture of the polarization (arrows) in a freely-suspended fluid ferroelectric film one molecular layer thick.