X-ray Diffraction Facility
A custom-built wide angle x-ray scattering (WAXS) system with a Huber 4-circle goniometer capable of accommodating large sample chambers (such as temperature-controlled ovens), this diffractometer is used routinely for structural characterization of liquid crystalline, polymeric and biological materials, x-ray reflectivity measurements on thin films, and high-resolution powder diffraction of polycrystalline materials.
High Resolution Electron Microscopy and Surface Structure Facility
This facility provides unique equipment to investigate the atomic scale structure of both the surface and sub-surface region of a sample combined with in situ growth and chemical characterization.
Surface Analysis and nano-Scale Imaging in the Micron Technology Foundation, Inc. Microscopy Suite
This USTAR-funded facility includes optical, electron, X-ray and ion microscopes, along with specialized analysis techniques to study surface topography, surface chemistry and even optical and dielectric properties of materials. The suite co-locates the MRSEC shared user facilities, the instruments of the Surface Analysis Lab, and also the Health Sciences Center (HSC) Electron Microscopy Core (EM for clinical pathology). Professional staff support includes PhDs in engineering, physics (laser optics, electron optics), physical & analytical chemistry, and cellular biology.
The ~5,300 square foot microscopy suite is the nexus point in the multidisciplinary facility, being strategically and metaphorically placed as a bridge between the engineering / science campus, and the medical school. Within the building, it is located between the research tower and the cleanroom. The microscopy suite is where these diverse researchers from all over campus physically bump into each other in a suite of tools designed to serve both hard and soft materials. This is where problem-solving engineers and life science researchers working on micro-and nano-scale devices meet life scientists who have ideas for quality-of-life-improving applications; but who need engineered solutions. We engineer having these people run into each other, and look for the magic that results!
LRSM Computing Facility
Location: South Bank Data Center
Supervisor/Coordinator: Robert A. Riggleman, Chemical and Biomolecular Engineering; Andrea J. Liu, Physics and Astronomy
Contact: Daniel Widyono
Email: [email protected]
Rob Riggleman, [email protected]

The LRSM Computing Facility is part of the Walnut High Performance Computing cluster (walnut.pics.upenn.edu) and is a shared facility for theory, simulation, and data analysis for projects supported by the MRSEC. The cluster consists of 62 nodes, each with four Intel E5-4620 8-core processors (at 2.2 or 2.6GHz) and 64GB RAM. The nodes are connected by a low-latency Infiniband network to allow for efficient parallelization. The MRSEC owns approximately 30% of the Walnut Cluster, giving MRSEC users priority access to 568 compute cores, and limited access to the entire cluster during periods of reduced utilization.
Rheology Center
Location: LRSM Building
Supervisor/Coordinator: Paul A. Janmey, Karen I. Winey, Paulo Arratia and Arjun G. Yodh
Equipment:
Confocal Rheometer
Faculty advisor: Paulo E. Arratia The LRSM possess a state-of-the-art confocal rheometer microscope equipped with a confocal microscope (Leica Stellaris) and a stress-controlled rheometer (TA Instrument DHR-3). This apparatus allows researchers to impose precise kinematic deformation onto a desired material while simultaneously characterizing the fluid microstructure and measuring the bulk fluid response.
The confocal microscope (Leica Stellaris) works by packaging a variable wavelength laser and a photo-multiplier detector into a single unit. With high precision, rapid motion of the objective (above 8KHz), achieved by harnessing a piezoelectric element, three dimensional (3D) scans (256 x 256 x 100 voxels) of a material’s structure can be created. Of key importance is that the scan be completed more rapidly than the time scales of imposed stress from the rheometer, ensuring minimal sample motion during the scan.
A TA Instrument DHR-3 rheometer is integrated to the confocal microscope. This hybrid rheometer is amenable to imaging via an off-the-shelf accessory and in-house optical manipulation. The DHR-3 provides good measurement quality for a variety of rheological tests and can resolve a torque range of 0.5nN.m to 200mN.m, with changeable geometries adding another two orders of magnitude to the range of detectable stresses. This is an important feature in providing enough dynamical rage from low to high viscosity samples.
TA Instruments RFS II
 Faculty advisor: Paul A. Janmey This facility is capable of characterizing both the structure and flow properties of simple and complex fluids. It can solve the most demanding fluid rheology problems including dynamic oscillatory and shear rate controlled measurements on low viscosity structured fluids. It is equipped with:
Faculty advisor: Paul A. Janmey This facility is capable of characterizing both the structure and flow properties of simple and complex fluids. It can solve the most demanding fluid rheology problems including dynamic oscillatory and shear rate controlled measurements on low viscosity structured fluids. It is equipped with:
- Peltier plate temperature control (from -30oC to 150 oC)
- Recirculating fluid bath (from -10 oC to 140 oC)
- Forced convection oven
- Torque ranges from 0.002 to 1000 g.cm
- Frequency ranges from 10-5 to 500 rad/sec
Bohlin Gemini
 Faculty advisor: Arjun G. Yodh, facility contact: Somayeh Farhadi
Faculty advisor: Arjun G. Yodh, facility contact: Somayeh Farhadi
The Bohlin Gemini rheometer is optimized for both stress controlled and strain controlled measurements. Technical features include a broad torque range, which extends to 200 mNm. The high-resolution torque mapping system applied to Bohlin’s low bias air bearing technology allows low torques to be set extremely accurately. The Bohlin is ideal for stress or strain controlled measurements of viscoelastic fluids with shear modulus as low as 1mPa. Couple with Nikon Eclipse 200/Confocal VT Eye microscopy for in-situ imaging during shearing (see details below)
Haake CaBER
 Faculty advisor: Arjun G. Yodh, facility contact: Somayeh Farhadi
Faculty advisor: Arjun G. Yodh, facility contact: Somayeh Farhadi
The Haake CaBer (capillary break-up extensional rheometer) provides valuable information about a material’s extensional properties that rotational rheometers cannot provide. With the CaBER, stringiness, filaments break-up time and extensional viscosity can be quantified.
Nikon Eclipse 200/Confocal VT Eye
Faculty advisor: Arjun G. Yodh, facility contact: Somayeh Farhadi
Inverted light microscope, capable of fluorescence microscopy. This microscope is attached to a VisiTech ‘VT-Eye’ confocal setup, and Bohlin Gemini rheometer. VisiTech ‘VT-Eye’
- 30 images per second (512 x 512 pixels); up to 400 images/s for reduced field of view
- Ultra-fast 3D acquisition: 256 x 256 x 100 pixel 3D image, 1 per second
- Z-scan up to 100 microns with 100 nm resolution
- Multi- wavelength excitation laser, at 488, 569 and 633 nm
- Reflection mode capability
- VoxCell software for easy control and data management
Instron Model 5564 Table Mounted Materials Testing System
 Faculty advisor: Karen I. Winey This facility is capable of performing tensile, compression, peel, and flexural tests on most materials and components. It is equipped with:
Faculty advisor: Karen I. Winey This facility is capable of performing tensile, compression, peel, and flexural tests on most materials and components. It is equipped with:
- 2 tension/compression load cells with capacity of 2kN and 10N, respectively.
- 1 rigid coupling.
- 1 micro 3-point bend fixture.
- 2 stainless steel tensile grips with 100lb capacity.
- 1 double-walled saline immersion vessel with digital temperature controller.
Keck Laboratory for Combinatorial Nanosynthesis and Multiscale Characterization
This powerful new facility was established under the support of the W. M. Keck Foundation. Combinatorial laser molecular MBE in the Keck Laboratory is used to make thin films of complex magnetic oxides. Materials are synthesized by atomic layer-by-layer deposition processes. The RHEED (reflection of high energy electron diffraction) oscillation is used to monitor the construction of materials unitcell by unitcell during the deposition. The combinatorial approach allows us to systematically fine tune the composition and process parameters of various thin film materials of interest. A state-of-the-art microwave microscope is being developed to provide measurement capabilities for various physical properties as well as imaging of materials at nanometer level. This facility is located in the new Jeong H. Kim Engineering and Applied Science Building; a virtual tour of the facility is available online. The Keck Laboratory is operated jointly with the Department of Material Science and Engineering as part of the Maryland Center for Integrated Nanoscale Science and Engineering.
UMass Computing Facility
This laboratory performs pioneering research into polymer structure and dynamics using the full array of modern computational methods (Monte Carlo, molecular dynamics, Brownian dynamics, various numerical procedures for solving nonlinear differential equations, etc.). The current simulations deal with pattern recognition by macromolecules, complex fluids, frustrated liquid crystals by confinement, kinetics of phase separation in polymer systems, and dynamics of polyelectrolytes in topologically controlled media with external fields. The shift in computational focus, as localized access to powerful workstations superseded remote access to supercomputers, has resulted in this laboratory's acquisition of its own set of computers. This includes five Digital DEC Alpha stations, two Silicon Graphics workstations and one Digital DEC Alpha server, which is a high performance processing unit. This array allows detailed atomistic computations of specific polymers.
MRSEC funds a computer facility under the supervision of Dr. Andre Melcuk. Resources available to the PSE community include a computer lab for first year students, and a document room for printing needs. The first year students' lab can house up to 25 students and contains 11 computers, a network printer and a photocopier. The document room contains a large-format HP Deisgnjet 2500CP printer, an HP Laserjet 4550 color printer, 1 Mac and 1 PC, an assrotment of graphics and presentation software, scanners, a CD-RW drive, and basic video editing hardware and software. The large-format HP 2500CP printer can print on media up to 36" wide and is available for poster printing.
In addition to the maintaining the above equipment, the MRSEC-funded computer support staff (Andre Mel'cuk and Sefa Nkrumah) maintains the department's network, ensures the proper operation of the research equipment within the department and is available for technical support and computer-related special projects.
Optical Imaging and Micromanipulation
Location: Room 333 LRSM Building
Coordinators: Prof. Arjun Yodh
 The center is a facility equipped with a wide array of optical microscopy and micro-manipulation systems to meet the needs of structural, dynamical and material characterization of soft matter, including colloids, emulsions, vesicles, liquid crystals and biomolecular materials. The facility contains:
The center is a facility equipped with a wide array of optical microscopy and micro-manipulation systems to meet the needs of structural, dynamical and material characterization of soft matter, including colloids, emulsions, vesicles, liquid crystals and biomolecular materials. The facility contains:
- Five optical microscopes equipped with high resolution digital video acquisition systems capable of bright field, phase contrast, high speed, and fluorescence microscopy.
- Three high-speed confocal setups, which can be used to study three-dimensional structure of materials, and biomolecular samples.
- One holographic tweezer system based on a Zeiss Axiovert 135 employs focused laser beams to manipulate microscopic objects. A spatial light modulator can generate complex patterns, and enables three-dimensional manipulation.
In addition to microscopy, the center provides other supporting optics equipment that is useful for characterization of soft materials. In particular, the center hosts a laser light scattering apparatus which is useful for angle-resolved static light scattering and photon correlation spectroscopy (i.e. quasi-elastic or dynamic light scattering spectroscopy).
EQUIPMENT:
Leica DMRX
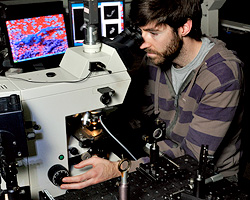 Upright light microscope, capable of bright field, dark field, polarization, phase contrast, fluorescence, differential interference contrast (DIC) microscopy, translational stage with position reader, and objective temperature control.
Upright light microscope, capable of bright field, dark field, polarization, phase contrast, fluorescence, differential interference contrast (DIC) microscopy, translational stage with position reader, and objective temperature control.
- 60 fps b/w digital CCD camera (0.32 MP) (UNIQ)
- mercury lamp for fluorescence measurements
- room 333
Leica DMIR13/VT-Eye Confocal
 Inverted light microscope, capable of bright field, dark field, polarization, phase contrast, fluorescence microscopy, with objective temperature control. This microscope is attached to a VisiTech ‘VT-Eye’ confocal setup VisiTech ‘VT-Eye’
Inverted light microscope, capable of bright field, dark field, polarization, phase contrast, fluorescence microscopy, with objective temperature control. This microscope is attached to a VisiTech ‘VT-Eye’ confocal setup VisiTech ‘VT-Eye’
- 30 images per second (512 x 512 pixels); up to 400 images/s for reduced field of view
- Ultra-fast 3D acquisition: 256 x 256 x 100 pixel 3D image, 1 per second
- Z-scan up to 400 microns with 100 nm resolution
- Multi- wavelength excitation laser, 488, 514 and 568 nm
- VoxCell software for easy control and data management
- 60 fps b/w digital CCD camera (0.32 MP) (UNIQ)
- room 333
Zeiss Axiovert 135/Optical Tweezer
 Inverted light microscope, capable of bright field, dark field, polarization, phase contrast, fluorescence microscopy with objective temperature control. This microscope is attached to a holographic tweezer setup with spatial light modulator (SLM), capable of generating complex patterns at up to 60 frames per second with 1064 nm infrared laser
Inverted light microscope, capable of bright field, dark field, polarization, phase contrast, fluorescence microscopy with objective temperature control. This microscope is attached to a holographic tweezer setup with spatial light modulator (SLM), capable of generating complex patterns at up to 60 frames per second with 1064 nm infrared laser
- fast (500 fps at ~1.3 MP, faster at reduced area of interest) b/w digital CMOS camera (Mikrotron)
- high-resolution (10 fps at 5 MP) b/w digital CMOS camera (EPIX)
- high-resolution (5 fps at 10 MP) b/w digital CMOS camera (EPIX)
- optical tweezers setup (Yodh group, NOT set up for general use)
- room 314
Brookhaven Instruments, BI-200SM, dynamic light scattering Capable of averaging, time-integrated intensity (classical) light scattering measurements, temperature control from 5˚C to 80˚C with stability of ±0.1 ˚C, angle selection with 0.01˚ steps
- red HeNe-laser, 15 mW
- currently not set up for static light scattering
- room 314
Leica DMRX A2 (Spinning Disk Confocal equipment available)
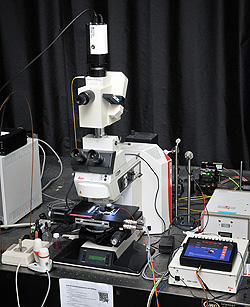 Upright light microscope, capable of bright field, dark field, polarization, DIC, and fluorescence contrast, fully automated with motorized stage (all three dimensions), and objective temperature control.
Upright light microscope, capable of bright field, dark field, polarization, DIC, and fluorescence contrast, fully automated with motorized stage (all three dimensions), and objective temperature control.
- 60 fps b/w digital CCD camera (0.32 MP) (UNIQ)
- equipment available, but not set up:
Visitech QLC-100 Spinning Disk Confocal setup for confocal imaging with excitation wavelength of 532nm, from external CrystaLaser diode pumped crystal laser source. MetaMorph software for control and data management.
- room 312
Nikon Eclipse 200/Confocal VT Eye
 Inverted light microscope, capable of fluorescence microscopy. This microscope is attached to a VisiTech ‘VT-Eye’ confocal setup, and Bohlin Gemini rheometer (Rheology Center) VisiTech ‘VT-Eye’
Inverted light microscope, capable of fluorescence microscopy. This microscope is attached to a VisiTech ‘VT-Eye’ confocal setup, and Bohlin Gemini rheometer (Rheology Center) VisiTech ‘VT-Eye’
- 30 images per second (512 x 512 pixels); up to 400 images/s for reduced field of view
- Ultra-fast 3D acquisition: 256 x 256 x 100 pixel 3D image, 1 per second
- Z-scan up to 100 microns with 100 nm resolution Multi- wavelength excitation laser, at 488, 569 and 633nm
- Reflection mode capability VoxCell software for easy control and data management
Light scattering facility
 Brookhaven Instruments, BI-200SM Capable of averaging, time-integrated intensity (classical), and intensity fluctuations (Quasi-elastic) light scattering measurements, temperature control from 5 ˚C to 80 ˚C with stability of ±0.1 ˚C, angle selection with 0.01˚ steps.
Brookhaven Instruments, BI-200SM Capable of averaging, time-integrated intensity (classical), and intensity fluctuations (Quasi-elastic) light scattering measurements, temperature control from 5 ˚C to 80 ˚C with stability of ±0.1 ˚C, angle selection with 0.01˚ steps.
Texas Materials Institute
The Texas Materials Institute (TMI) operates the Materials Science and Engineering graduate program, provides the instrumentation necessary to conduct modern materials research, and promotes interdisciplinary research in materials science and engineering.
Showing 1901 to 1910 of 2586